Spdf Orbitals. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. Helium, has two protons and two neutrons that comprise the. It discusses the 4 quantum numbers n, l, ml, and ms. This video explains s, p, d, and f orbitals, sublevels, and their shapes. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. These orbitals have different shapes (e.g. At the first energy level, the only orbital available to electrons is the. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. For k shell there is only one s subshell. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The orbitals are of 4 types. Spdf orbitals are energy sub level or sub shells. N represents the energy level. The letters and words refer to the visual impression left by the fine.
Spdf Orbitals : Normal Basis Set Atomic Orbitals.
Electron Configuration Chart. This video explains s, p, d, and f orbitals, sublevels, and their shapes. N represents the energy level. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. It discusses the 4 quantum numbers n, l, ml, and ms. For k shell there is only one s subshell. Spdf orbitals are energy sub level or sub shells. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. At the first energy level, the only orbital available to electrons is the. The orbitals are of 4 types. The letters and words refer to the visual impression left by the fine. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. These orbitals have different shapes (e.g. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. Helium, has two protons and two neutrons that comprise the.
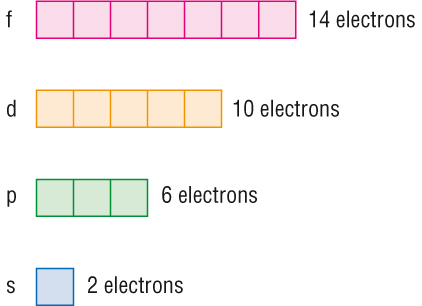
This video explains s, p, d, and f orbitals, sublevels, and their shapes.
But i'm sorta curious to know that what is spdf configuration and. Find out information about spdf. Contribute to kshaa/orbitals development by creating an account on github. This number divides the shells into subshells (also called sublevels or orbitals). At the first energy level, the only orbital available to electrons is the. N represents the energy level. It by spdf configuration, he meant orbital configuration. In chemistry, spdf are the names of the orbitals. Recently in my chemistry classes, the teacher spoke about spdf configuration and then said that we'll be taught about it in higher classes. S stands for sharp, p for principle, d for diffuse and f for fundamental. For historical reasons the designated letters stand for sharp, principal, diffuse, and fundamental, based on the spectral line observations associated with. The orbitals are of 4 types. Learn vocabulary, terms and more with flashcards, games and other study tools. For k shell there is only one s subshell. Now the basic of this concept is from very. Normal basis set atomic orbitals. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. .p orbitals, d orbitals and f orbitals ñ the 'spaces' in which an electron occupied quantum level spdf notation for 53 iodine, i ?, spdf notation for 54 xenon, xe ?, spdf notation for 55 caesium, cs. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. Struggling with electronic configuration and spdf notation in prelim chemistry? From wikimedia commons, the free media repository. Helium, has two protons and two neutrons that comprise the. It discusses the 4 quantum numbers n, l, ml, and ms. A wide variety of d electron orbitals options are. Negatively charged electrons are attracted to a positively charged nucleus to form an atom or ion. Other orbitals include the p, d and f orbitals. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. Can anyone explain to me in laymans terms the whole s p d f business? The letters and words refer to the visual impression left by the fine. The orbital arrangement of an atom's electrons.
2 4 Electron Configurations Chemistry Libretexts , Helium, Has Two Protons And Two Neutrons That Comprise The.
Visualizing Electron Orbitals. It discusses the 4 quantum numbers n, l, ml, and ms. At the first energy level, the only orbital available to electrons is the. The orbitals are of 4 types. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. For k shell there is only one s subshell. Helium, has two protons and two neutrons that comprise the. N represents the energy level. This video explains s, p, d, and f orbitals, sublevels, and their shapes. These orbitals have different shapes (e.g. Spdf orbitals are energy sub level or sub shells. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The letters and words refer to the visual impression left by the fine. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals.
Orbital Diagrams Ppt Download - .P Orbitals, D Orbitals And F Orbitals Ñ The 'Spaces' In Which An Electron Occupied Quantum Level Spdf Notation For 53 Iodine, I ?, Spdf Notation For 54 Xenon, Xe ?, Spdf Notation For 55 Caesium, Cs.
Electron Configurations Orbitals Energy Levels And Ionisation Energy Trends A Level Chemistry Revision Notes. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. This video explains s, p, d, and f orbitals, sublevels, and their shapes. Helium, has two protons and two neutrons that comprise the. Spdf orbitals are energy sub level or sub shells. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. The orbitals are of 4 types. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. It discusses the 4 quantum numbers n, l, ml, and ms. These orbitals have different shapes (e.g. N represents the energy level.
Classification Of Elements Into S P D F Blocks In The Periodic Table , S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape.
Quantum Model And Spdf Orbitals Youtube. These orbitals have different shapes (e.g. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. The orbitals are of 4 types. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. N represents the energy level. For k shell there is only one s subshell. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. This video explains s, p, d, and f orbitals, sublevels, and their shapes. Helium, has two protons and two neutrons that comprise the. At the first energy level, the only orbital available to electrons is the. Spdf orbitals are energy sub level or sub shells. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. The letters and words refer to the visual impression left by the fine. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. It discusses the 4 quantum numbers n, l, ml, and ms.
Electronic Structure And Periodic Table Mcat Review . S Orbitals Have 2 Vectors P Orbitals Have 6 Vectors D Orbitals Have 14 Vectors F Orbitals Have 14 Orbitals And Nuclear Shape.
Quantum Numbers N L Ml Ms Spdf Orbitals Youtube. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. N represents the energy level. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. It discusses the 4 quantum numbers n, l, ml, and ms. This video explains s, p, d, and f orbitals, sublevels, and their shapes. For k shell there is only one s subshell. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. The orbitals are of 4 types. Helium, has two protons and two neutrons that comprise the. At the first energy level, the only orbital available to electrons is the. These orbitals have different shapes (e.g. The letters and words refer to the visual impression left by the fine. Spdf orbitals are energy sub level or sub shells.
If Each Orbital Can Hold A Maximum Of 3 Electrons The Number Of Elements In The 4th Bb Period Of The Periodic Table Long Form Is Socratic . But I'm Sorta Curious To Know That What Is Spdf Configuration And.
Powerpoint Orbital Shape Orientation. These orbitals have different shapes (e.g. Helium, has two protons and two neutrons that comprise the. Spdf orbitals are energy sub level or sub shells. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. For k shell there is only one s subshell. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. At the first energy level, the only orbital available to electrons is the. The orbitals are of 4 types. This video explains s, p, d, and f orbitals, sublevels, and their shapes. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. The letters and words refer to the visual impression left by the fine. N represents the energy level. It discusses the 4 quantum numbers n, l, ml, and ms. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape.
Ib Chemistry Topic 12 1 Electronic Configuration - Atomic Orbitals Are The Basic Building Blocks Of The Atomic Orbital Model (Alternatively Known As The Electron Cloud Or Wave Mechanics Model), A Modern Framework For Visualizing The Submicroscopic.
Electron Configuration Wikipedia. For k shell there is only one s subshell. This video explains s, p, d, and f orbitals, sublevels, and their shapes. N represents the energy level. The letters and words refer to the visual impression left by the fine. At the first energy level, the only orbital available to electrons is the. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. These orbitals have different shapes (e.g. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. The orbitals are of 4 types. Spdf orbitals are energy sub level or sub shells. Helium, has two protons and two neutrons that comprise the. It discusses the 4 quantum numbers n, l, ml, and ms.
S P D F Orbitals And Angular Momentum Quantum Numbers : When The Spdf Orbitals Are Subjected To A 3D, Visual Summing, They Present Anything But A Neat Assemblage Of The Electron Spaces Needed To Handle The Required Number Of Periodic Electrons.
Shapes Of The 3d Orbitals In 3d. For k shell there is only one s subshell. This video explains s, p, d, and f orbitals, sublevels, and their shapes. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. The letters and words refer to the visual impression left by the fine. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. At the first energy level, the only orbital available to electrons is the. The orbitals are of 4 types. It discusses the 4 quantum numbers n, l, ml, and ms. These orbitals have different shapes (e.g. N represents the energy level. Helium, has two protons and two neutrons that comprise the. Spdf orbitals are energy sub level or sub shells. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape.
File Atomic Orbital Clouds Spdf M0 Png Wikipedia . S Orbitals Have 2 Vectors P Orbitals Have 6 Vectors D Orbitals Have 14 Vectors F Orbitals Have 14 Orbitals And Nuclear Shape.
Bohr Model S P D F Orbitals Flashcards Quizlet. This video explains s, p, d, and f orbitals, sublevels, and their shapes. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. For k shell there is only one s subshell. At the first energy level, the only orbital available to electrons is the. N represents the energy level. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. These orbitals have different shapes (e.g. It discusses the 4 quantum numbers n, l, ml, and ms. Helium, has two protons and two neutrons that comprise the. The orbitals are of 4 types. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. Spdf orbitals are energy sub level or sub shells. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. The letters and words refer to the visual impression left by the fine.
Orbitals Lecture Notes 6 Chem 6a General Chemistry I Studocu , At The First Energy Level, The Only Orbital Available To Electrons Is The.
Quantum Number Wikipedia. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic. N represents the energy level. The letters and words refer to the visual impression left by the fine. These orbitals have different shapes (e.g. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. At the first energy level, the only orbital available to electrons is the. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. Helium, has two protons and two neutrons that comprise the. It discusses the 4 quantum numbers n, l, ml, and ms. For k shell there is only one s subshell. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. This video explains s, p, d, and f orbitals, sublevels, and their shapes. Spdf orbitals are energy sub level or sub shells. The orbitals are of 4 types.
Figure A 1 Angular Dependence Of The S P D And F Orbitals With L Download Scientific Diagram : Other Orbitals Include The P, D And F Orbitals.
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes. These orbitals have different shapes (e.g. At the first energy level, the only orbital available to electrons is the. Spdf orbitals are energy sub level or sub shells. An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball not all electrons inhabit s orbitals. N represents the energy level. For k shell there is only one s subshell. Helium, has two protons and two neutrons that comprise the. They are named s,p,d,f.the s, p, d, and f stand for sharp, principal, diffuse and fundamental, respectively. The orbitals are of 4 types. It discusses the 4 quantum numbers n, l, ml, and ms. The letters and words refer to the visual impression left by the fine. This video explains s, p, d, and f orbitals, sublevels, and their shapes. S orbitals have 2 vectors p orbitals have 6 vectors d orbitals have 14 vectors f orbitals have 14 orbitals and nuclear shape. When the spdf orbitals are subjected to a 3d, visual summing, they present anything but a neat assemblage of the electron spaces needed to handle the required number of periodic electrons. Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic.